|
|
|
Threshold Values: pH |
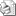
|
|
In ponds pH is ideally regulated by two buffering systems. These can, however, take some time to start working.
That is why in newly constructed ponds there can be strong variations:
The Calcium/Carbonic Acid System
In the starting phase ponds are mostly unbuffered and pH can vary strongly.
The calcium/carbonic acid system is therefore only theoretically present in new ponds because hardly any decomposition takes place that could set free carbon dioxide.
This automatically results in a lack of carbonic acid. Both components, however, are prerequisites for buffering and stabilising pH at, say, 7.2.
_
|
Movement Sets CO₂ Free
In ponds that are built and operated in a very non-natural way, the development of carbonic acid is mostly prevented. Next to intense water movement through the filtration system, any existing CO₂ is eliminated through aeration. This can lead to high pH between 7.5 and 8. An effective filtration system that eliminated any organic residue makes the problem worse under these circumstances, because it removes the last source of CO₂.
In ponds with many plants and/or algae pH values up to 11 can be reached, because these also extract CO₂ from the water.
|
 |
The Humic Acid System
In natural ponds, decomposed plant residue forms humic acids that stabilise the water around pH 5.5. Both systems together result in pH values of 6.5 - 7, the ideal range.
With efficient filtration systems that prevent all composting processes the formation of humic acids is stopped, too, so that such ponds often have a too high pH level and fish are in danger of ammonia poisoning. |
Effects
In over-filtered ponds, pH has to be checked regularly. If it is too high and due to the small volumes of water, it can be lowered using peat as a filtering medium. The contact with peat washes out humic acids that lower pH.
With low pH the concentration of nitrous acid, that forms from existing nitrite, rises. In the fish itself the nitrite is freed, often reaching 70 times the concentration in the water. The outlook here is bleak... |
 |
|
|